What does writing your PhD have to do with Shiny Apps?
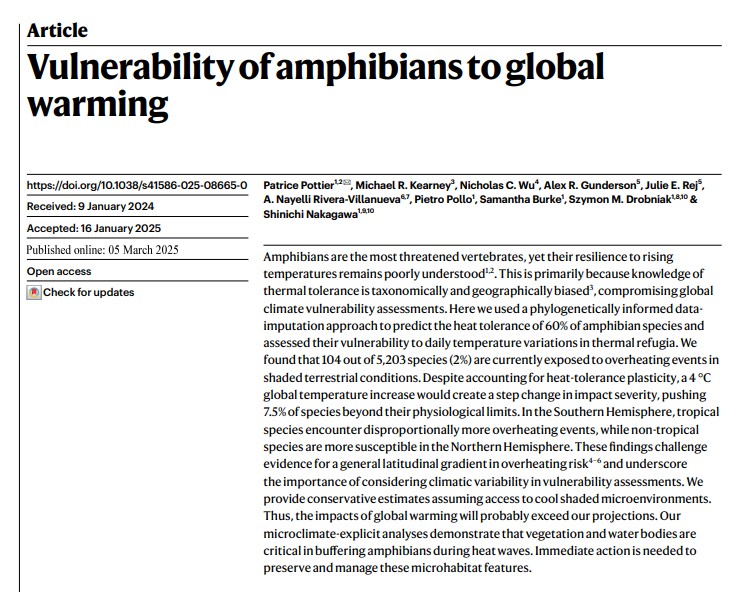
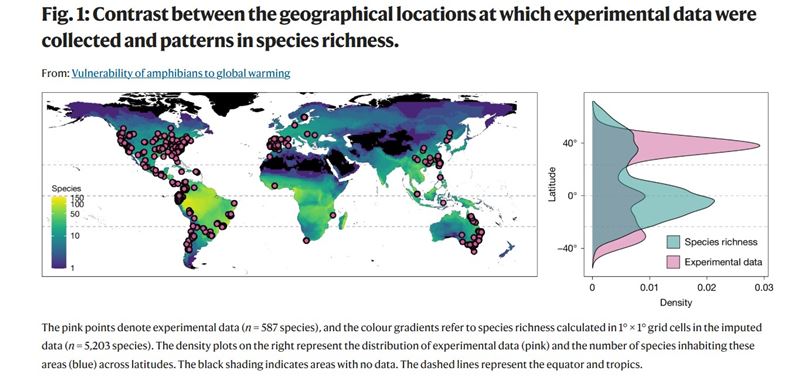
Shiny apps are R based interactive web applications that sit on a Shiny server. They make it easy for users to create plots and tables through a series of sliders or dropdowns. How to Write a PhD is already an interactive book in that readers can request alterations and additions (see here). But the book itself was static - until now. In the past 2 weeks, I have started to insert Shiny Apps into chapters where they might enhance content.
The first example appears here in the chapter on Time Management. I know from my experience teaching students in my class on Writing Skills that although telling them that drawing a Gantt Diagram to plan their first year of studies sounds easy, they struggle with getting started. So here's a Shiny App that does just that:
Don't take my word for it - have a go yourself!
The other Shiny App explains in set theory how Boolean Operators work. Here it is:
Perhaps more controversially, the last Shiny App shows you how you could get started on writing the first paragraph of your discussion. An oportunity to get input from AI is also provdided together with a caution: Please remember You may use the AI generated text to help and inspire you to compose your own text, but do not copy and paste it. A full explanation of why this is not acceptable can be found in an earlier chapter on using Artificial Intelligence. Here it is:
If you like these Shiny Apps, and have an idea about which app should come next in How to Write a PhD or How to Publish Science, then let me know!